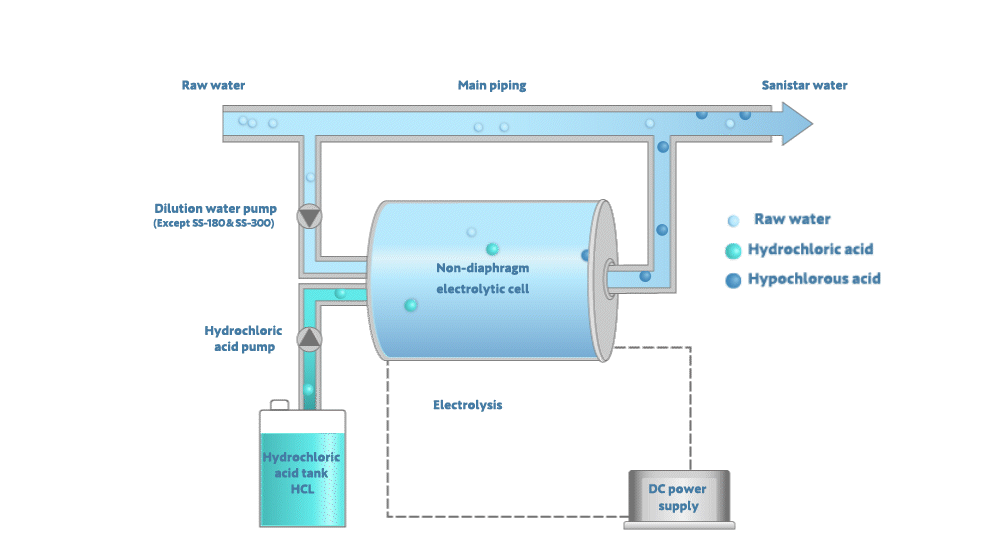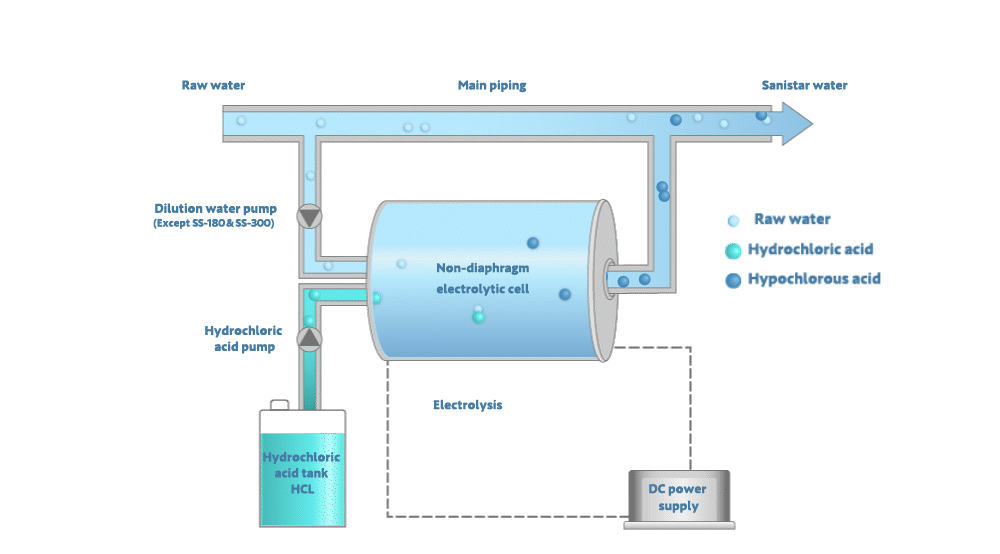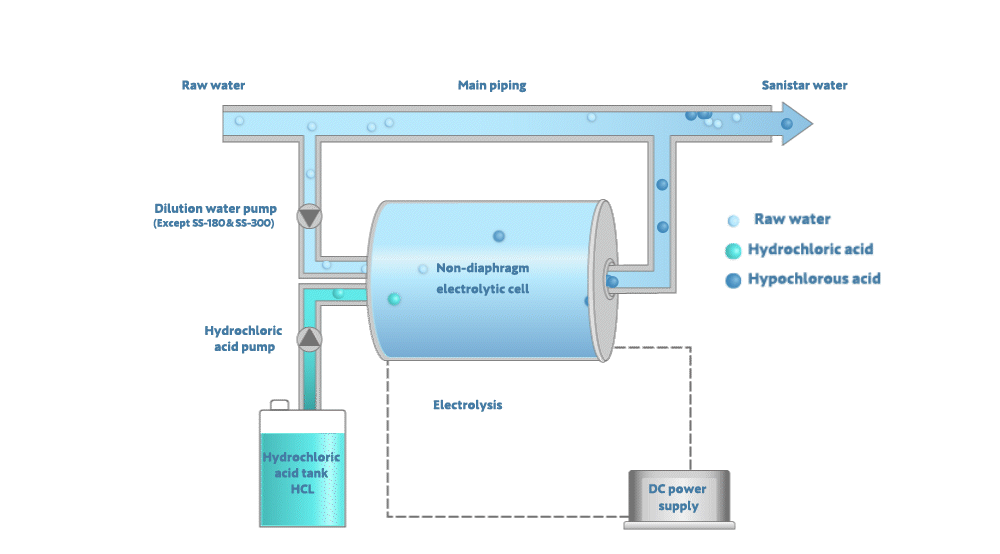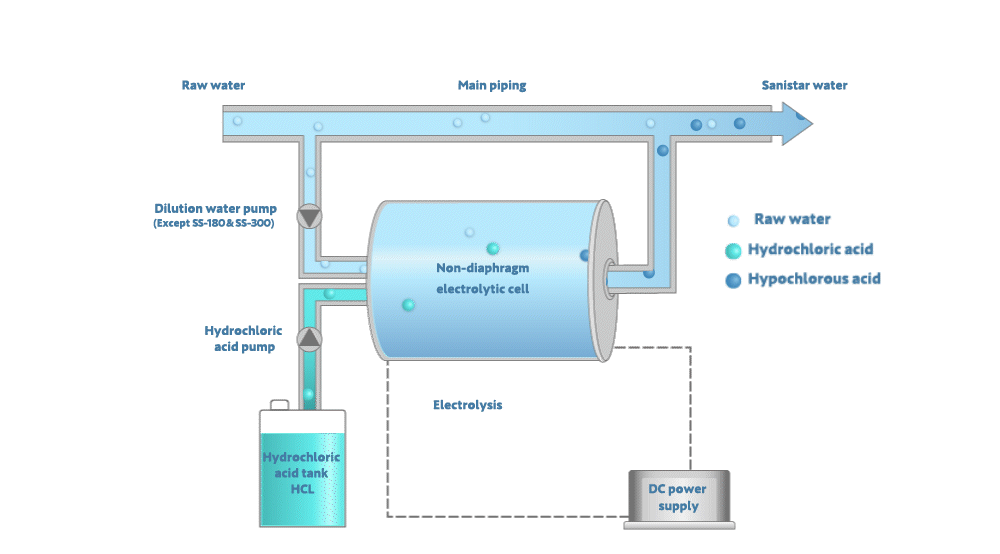
Electrolyzing the dilute hydrochloric acid generates Sanistar Water
The slightly acidic electrolyzed water is made from hydrochloric acid.
As shown in the figure below, when electrolyzing the dilute hydrochloric acid and water in a non-diaphragm electrolytic-cell, Sanistar Water is generated.
Low chlorine concentration but strong bactericidal power
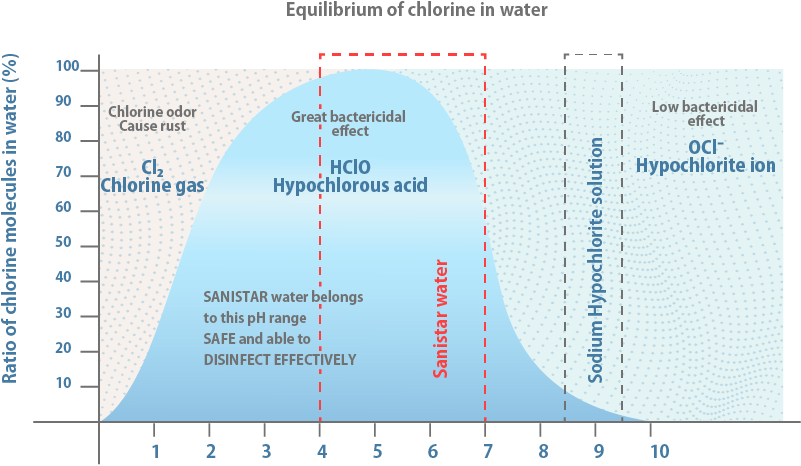
Sanistar Water shows strong bactericidal power at low chlorine concentration (10-30ppm*) as the ratio of hypochlorous acid is high.
It is almost colorless, tasteless and odorless. There is no problem if you touch or even swallow it.
* SS-180: 15~35ppm
Feature of Sanistar Water
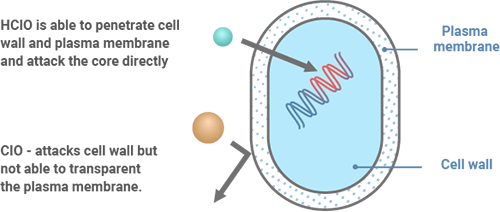
The disinfectant main component is HClO, which has about 100 times more disinfectant power than OCl-.
Sanistar Water has the same sanitation power as sodium hypochlorite at 1/10 of the concentration of active ingredients. Sanistar Water: available chlorine 10-30 ppm*, pH 4.0-7.0
* SS-180: 15~35ppm
Why it is Safe?
Hypochlorous acid water was approved as a food-additive disinfectant in 2002 in Japan. It is approved to apply for food materials and food-contact surfaces.
Less Odor & By-Product
Since Sanistar uses a low concentration, it does not have a strong chlorine odor compared to disinfectants such as sodium hypochlorite.
Sodium hypochlorite (NaClO) generates chlorates, which may cause oxidative damage to hemoglobin, such as sodium chlorate (NaClO3) during its oxidation reaction process NaClO + O2 → NaClO3(Chlorate)
The tap water quality standard for chlorate in Japan is 0.6mg/L or less. When the chlorate in Sanistar water was measured, it was below the detection limit. It means the chlorate in Sanistar water is equivalent to that in tap water.

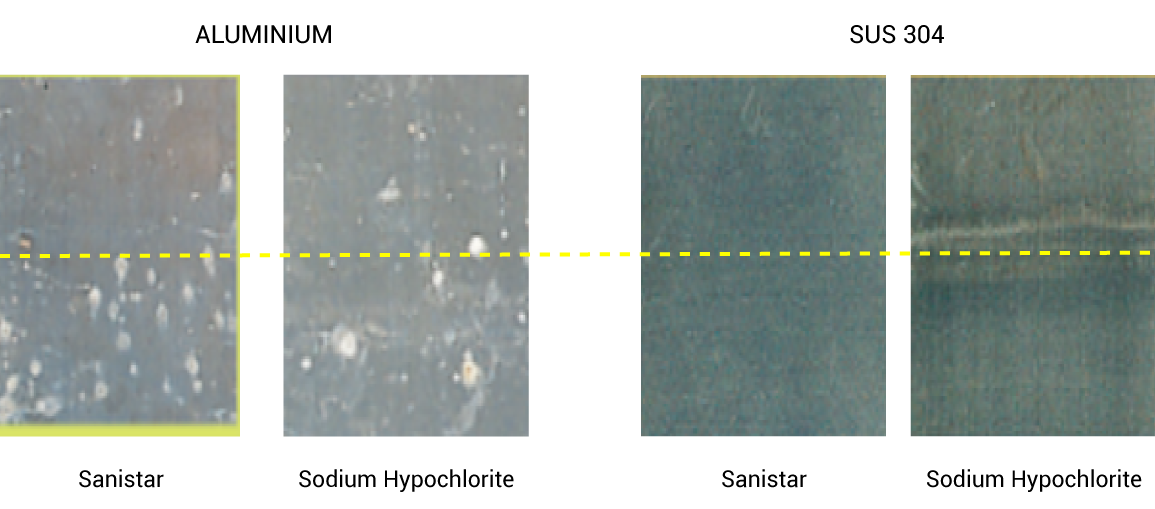
Less Damage To Your Facility
Sanistar water does not use salt as a raw material, rust is less likely to occur, so there is little impact on equipment, and rinsing is almost unnecessary.
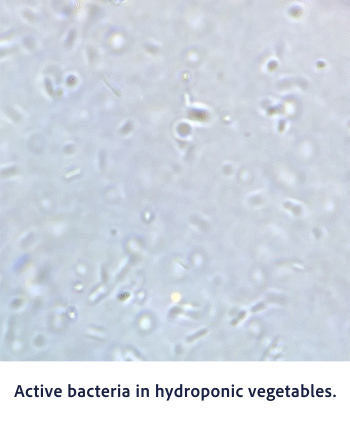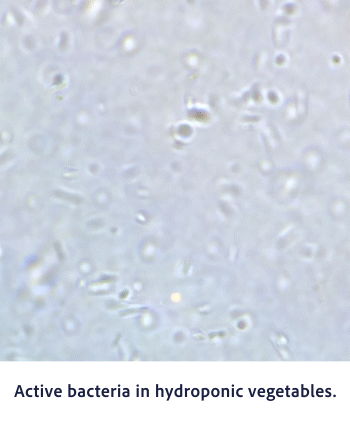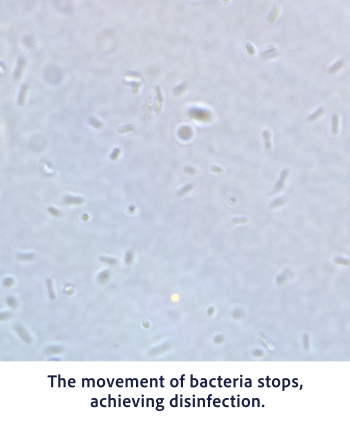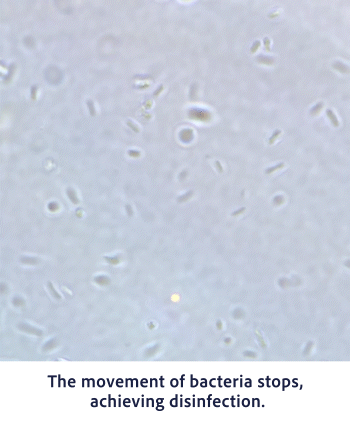
Bactericidal Efficacy
Hypochlorous acid (HClO) has a strong oxidizing effect. In the process of extracting electrons from other substances and becoming Cl-, it exhibits strong sterilization and deodorizing effects. This powerful oxidation reaction is the source of the disinfecting and deodorizing power.
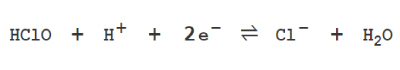
Why Does HClO Have a Stronger Bactericidal Efficacy Than ClO-?
Difference from Sodium Hypochlorite
| Sodium hypochlorite | Sanistar | |
|---|---|---|
| Convenience | Have to be diluted manually and check the concentration frequently | Just use as it is from the tap |
| Bactericidal Effect | There is some kind of bacteria which is not killed with 200ppm sodium hypochlorite | Effective for bacteria, virus and mold |
| Safty | Needed to wear gloves and protect your skin and eyes | Safe to contact skin, odorless and less corrosive |
| Usage | Soaking for long time and have to be rinse off | Just rinse the objects with running sanistar |
Cost Effectiveness
Without Sanistar
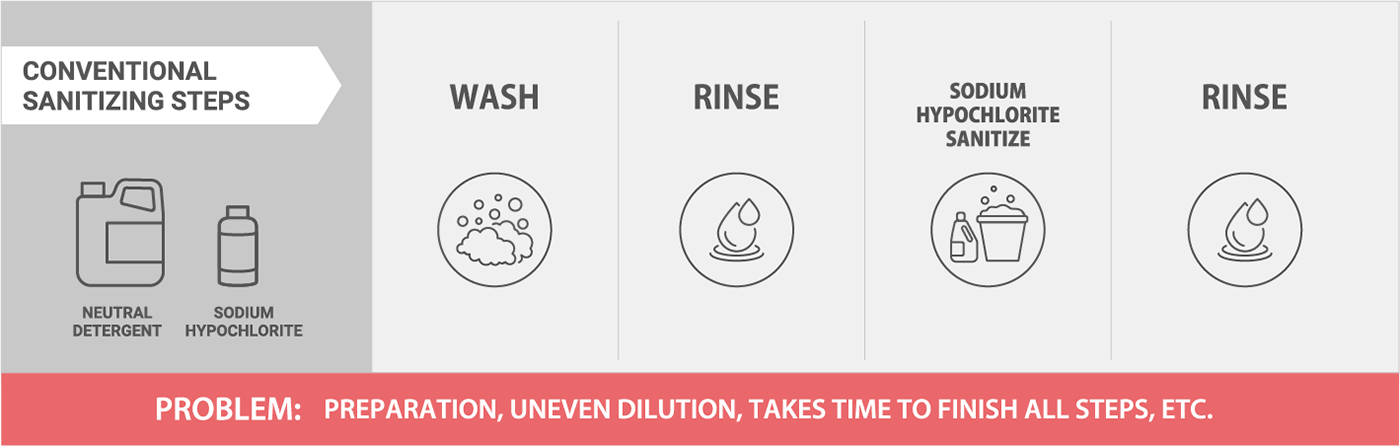
With Sanistar